42 in the sketch of the structure of nf3 label all bonds.
Solved In the sketch of the structure of BF3 label all | Chegg.com Expert Answer 100% (32 ratings) Transcribed image text: In the sketch of the structure of BF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. (PDF) general-chemistry.pdf | Sumit Banerjee - Academia.edu general-chemistry.pdf
Solved In the sketch of the structure of NF3 label all | Chegg.com Transcribed image text: In the sketch of the structure of NF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all.
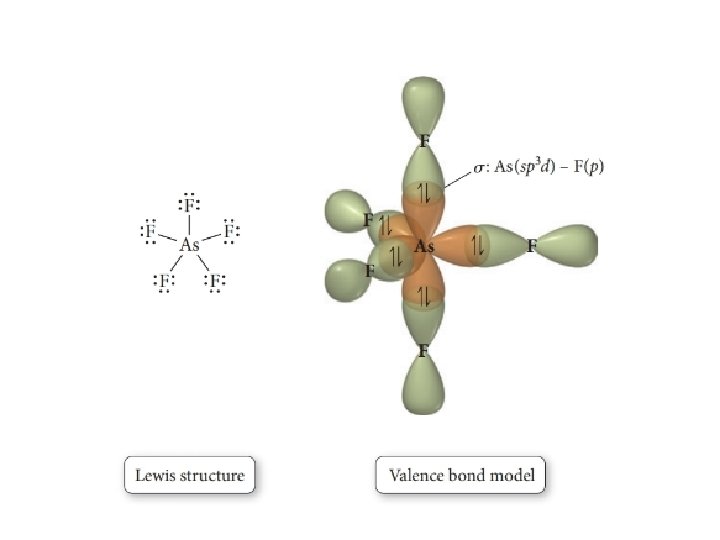
In the sketch of the structure of nf3 label all bonds.
SOLVED:Write a hybridization and bonding scheme for each molecule ... We drew two bonds, um, or two bonds to each oxygen, which takes four of its electrons, and it has a total of six. So we have an extra lone electron pair here. So adding up the total things on the sulfur we see that's using to pure metals, Um, and two Sigma or Bills and the lone electron pairs. So this has to be S P three d. CHEM: Chapter 10 Flashcards | Quizlet The skeletal structures of several simple amino acids are shown here. For each skeletal structure, complete the Lewis structure, determine the geometry and hybridization about each interior atom, and make a sketch of the molecule, using the bond conventions of Section 10.4. (On Doc) Cumulative Problem 92. Inorganic Chemistry 4th edition, Catherine Housecroft Electronic Structure and Spectro-Structural Correlations of Fe III Zn II Biomimetics for Purple Acid Phosphatases: Relevance to DNA Cleavage and Cytotoxic Activity. by Claus T Pich, HernÃn Terenzi, Rosely Peralta, Faruk Nome, and Eduardo Castellano.
In the sketch of the structure of nf3 label all bonds.. Use valence bond theory to write the hybridization and ... - Socratic Step 1. Draw the Lewis structure (a) Start with a skeleton structure. The two #"C"# atoms (least electronegative) will be the central atoms, with the #"N"# attached to one of the carbons. (b) Attach the hydrogen atoms. The question gives you a clue where they go. The formula #"NCCH"_3# tells you that the three #"H"# atoms are attached to the terminal carbon atom. NF3 lewis structure, molecular geometry, bond angle ... - Topblogtenz Simple steps for drawing the NF3 lewis dot structure 1. Count total valence electron in NF3 In the first step, we have to calculate the total number of valence electrons present in the NF3 molecule. Nitrogen is present in the 15th group in the periodic table and Fluorine in group 17th. ⇒ Total valence electron in Nitrogen = 5 NH3 Lewis Structure, Geometry, and Hybridization Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state. Ammonia is lighter than the air, colorless, and pungent in smell. Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in SO2. Label all bonds in NF3. Label all bonds in BF3. Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o C (sp') Br (s) C (p) H (p) C (p) Br (p) C (sp) H (p) o C (sps) Br (p) C (sps) Br (p) reset help Expert's Answer Solution.pdf
PDF Tro Chemistry - A Molecular Approach 2nd Edition Sketch the molecule, including overlapping orbitals, and la all bonds using the notation shown in Examples 10.6 and IC a. CH2Br2 b. c. d. BF3 Write a hybridization and bonding scheme for each molecul' ion. Sketch the structure, including overlapping orbitals, ; label all bonds using the notation shown in Examples 10.6 ; 10.7. a. Answered: ch D. In the sketch of the structure of… | bartleby In the sketch of the structure of NF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Reset Help Question Transcribed Image Text:MISSED THIS? KCV 11.7, IWE 11.8. Write a hybridization and bonding scheme for each molecule. tab esc os lock control POD FES ! 1 10 FI Q A Answered: In the sketch of the structure of SO2… | bartleby In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. : S (p) - O (p) Lone pair in p orbital Lone pair in sp? orbital o : S (p) - O (sp²) т: S (p) — О (p) т: S (sp?) Geometry Questions and Answers | Homework.Study.com The shape of a carbon atom as it bonds to four other ... NF3 C) OF2 D) H2S . View Answer. Dana takes a paper cone 12 cm in diameter with a 10 cm height, cuts it from the rim to the vertex, and flattens ... Find the level curves for f = 1, 2, -1. 2. Sketch and label each level curve. View Answer. Angle A is complementary to angle B and angle B ...
BF3 Lewis Structure, Molecular Geometry, Hybridization, and Polarity To draw a Lewis Structure, first of all, add electrons and draw the connectivities. As discussed, here there are 24 electrons. Then, add octets to the outer atom and extra electrons to the central atom. But, as we know, there are no extra electrons. (24 - 24 = 0) Violations NCl3 lewis structure, molecular geometry, bond angle ... - Topblogtenz NF3 lewis structure has 3 fluorine and 1 nitrogen atom connected with three single bonds and NH3 lewis structure has 3 hydrogens and 1 nitrogen connected with three single bonds also. In all these molecules(NH3, NF3, and NCl3), there is one lone pair present on the central atom. SOLVED:Draw the hybrid orbital diagram for each of the atoms in the ... Draw the hybrid orbital diagram for each of the atoms in the following molecules, then sketch each molecule. Label all hybrid orbitals (sp3 , sp2 , sp, s, p) and label all bonds as sigma or pi on the diagram. Hybridize all non-hydrogen atoms. 1. COCl2 2. CNCl 3. CH3SH SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Write a hybridization and bonding scheme for each molecule or ion. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. COCl2 (carbon is the central atom) b. BrF5 c. XeF2 d. I3
CH3F lewis structure, molecular geometry, bond angle ... - Topblogtenz Being the least electronegative, carbon is the central atom in CH3F lewis's structure. There is a total of 4 bonded pairs and 3 lone pairs present in the CH3F lewis dot structure. To draw the lewis dot structure of CH3F follows some simple steps. Step 1: In the first step, Count all valence electrons present in CH3F. For finding valence ...
Chapter 6, Chemical Bonding II Video Solutions, Chemistry: Structure ... Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. N2H2 (skeletal structure HNNH) b.
NH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle & Shape NH3 Bond angles There are three single bonds and one lone pair of electrons in the NH3 molecule. It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure. The shape is distorted because of the lone pairs of electrons. This pair exerts repulsive forces on the bonding pairs of electrons.
NBr3 lewis structure, molecular geometry, bond angle ... - Topblogtenz Yes, the lewis structure of NBr3 is almost the same as NF3 and NCl3. NF3 lewis structure has 3 fluorine and 1 nitrogen atom connected with three single bonds and NCl3 lewis structure has 3 chlorine and 1 nitrogen connected with three single bonds also. In all these molecules (NBr3, NF3, and NCl3), there is one lone pair present on the central atom.
Answered: Please draw the structure of psylocibin… | bartleby 16.8.2022 · Solution for Please draw the structure of psylocibin (C12H17N2O4P), with its ... State the type of hybrid of each carbon, label all the bonds involve. arrow_forward. Allene (1,2-propadiene), H2C=C=CH2, has two adjacent double bonds ... The nitrogen atom of NF3 is sp3-hybridized. The orbitals of an sp2-hybridized atom are located 120 ...
Answered: In the sketch of the structure of NF3… | bartleby Transcribed Image Text: In the sketch of the structure of NF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. σ: Ni) - F() Lone pair in sp orbital 1L o : N(p) - F(sp³) : N(sp³) - F(p) Lone pair in p orbital T: N(p) - F(p) Lone pair in s orbital σ: Nsp') - Ffp)
Stanford University UNK the , . of and in " a to was is ) ( for as on by he with 's that at from his it an were are which this also be has or : had first one their its new after but who not they have
Post Lecture Lewis Structures, Problem Set #6 (Ch 5), Problem ... - Quizlet Start studying Post Lecture Lewis Structures, Problem Set #6 (Ch 5), Problem Set #7 (Ch 6,7). Learn vocabulary, terms, and more with flashcards, games, and other study tools. ... Use the drawing of MO energy diagram to predict the bond order of Be2−. ... The molecule is polar because all the I−F bonds are polar and the net dipole moment is ...
Inorganic Chemistry 4th edition, Catherine Housecroft Electronic Structure and Spectro-Structural Correlations of Fe III Zn II Biomimetics for Purple Acid Phosphatases: Relevance to DNA Cleavage and Cytotoxic Activity. by Claus T Pich, HernÃn Terenzi, Rosely Peralta, Faruk Nome, and Eduardo Castellano.
CHEM: Chapter 10 Flashcards | Quizlet The skeletal structures of several simple amino acids are shown here. For each skeletal structure, complete the Lewis structure, determine the geometry and hybridization about each interior atom, and make a sketch of the molecule, using the bond conventions of Section 10.4. (On Doc) Cumulative Problem 92.
SOLVED:Write a hybridization and bonding scheme for each molecule ... We drew two bonds, um, or two bonds to each oxygen, which takes four of its electrons, and it has a total of six. So we have an extra lone electron pair here. So adding up the total things on the sulfur we see that's using to pure metals, Um, and two Sigma or Bills and the lone electron pairs. So this has to be S P three d.
Post a Comment for "42 in the sketch of the structure of nf3 label all bonds."